Now You See It, Now You Don’t: Making Biodegradable Plastics Disappear
By Laura Tran, C2ST Intern, Rush University
Biodegradables won’t solve our plastic crisis. Despite our efforts to recycle, the overall amount of plastic that is recycled is less than 9% in the U.S.¹. Many people do not recycle for several reasons, such as a lack of knowledge for what is recyclable and where to sort it, or they lack access to appropriate and convenient recycling facilities. To combat this issue, today’s biodegradable plastics are advertised as a solution to the world’s increasing plastic pollution problem. However, these materials come with their own challenges.
If biodegradable plastics are not properly sorted, they can contaminate other recyclable plastics and ultimately end up in a landfill. Even worse, these biodegradable plastics take months to break down into microplastics²—which are still plastic, just smaller, and can be potentially harmful to humans and the environment! So how can we clean up our act?
Scientists from the University of California, Berkeley³ have engineered a way for biodegradable plastics to break down more easily. Just add heat and water and you can break down these materials from the comfort of your own home! The idea is almost too good to be true, but how exactly does this process work on plastic?
Biodegradable plastics are usually made of two polymers called polylactic acid (PLA) and polycaprolactone (PCL). These biodegradable plastics are intended to break down when exposed to the environment (i.e., outside forces). However, the new and improved biodegradable plastic is embedded with polymer-eating enzymes. These enzymes are the driving forces for plastics to be broken down from the inside out!
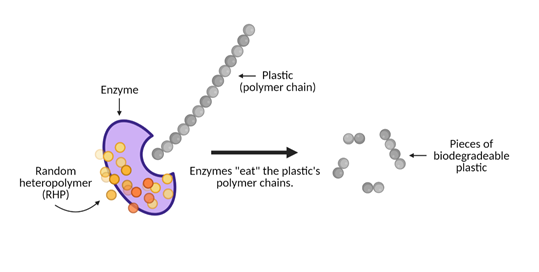
How do these polymer-eating enzymes work to make plastic break down?
Think of this breakdown process as dinnertime. The enzymes are the utensils that will break down the plastic. Two different enzymes that work within these biodegradable plastics are proteinase (which breaks down proteins) and lipase (which breaks down fatty acids). The utensils are wrapped in a napkin that protects the utensils from getting dirty, much like how random heteropolymers, or RHPs, protect the enzyme from breaking down and becoming unusable before it’s ready for “dinner time”.
Enzymes are exposed to 40 to 60°C heat (ah, just the right temperature) that breaks down the protective coating and reveals the enzyme’s active site. The active site serves as our dinner table. This is where “eating” occurs. The food in this scenario is the plastic, specifically the plastic’s polymer chains. When it is dinner time, the plastic’s polymer chains bind to the active site and the enzymes begin to chow down. It eats away at this plastic as though it were long strands of spaghetti and breaks down the plastic polymer chains into smaller pieces, or “monomers”.

Not only can we reduce biodegradable plastic in the ground, but scientists are working to use RHPs on other types of polymer plastics. But there is a twist! The goal is to program the degradation to stop at a specific point to repurpose the plastic into something else. This is how we take “reduce, reuse, and recycle” to a whole other level.
References
- Geyer, R., Jambeck, J. R., & Law, K. L. (2017). Production, use, and fate of all plastics ever made. Science advances, 3(7), e1700782. https://doi.org/10.1126/sciadv.1700782
- DelRe, C., Jiang, Y., Kang, P., Kwon, J., Hall, A., Jayapurna, I., … Xu, T. (2021). Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature, 592(7855), 558–563. https://doi.org/10.1038/s41586-021-03408-3
- Smith, M., Love, D. C., Rochman, C. M., & Neff, R. A. (2018). Microplastics in Seafood and the Implications for Human Health. Current environmental health reports, 5(3), 375–386. https://doi.org/10.1007/s40572-018-0206-z