A Look Back at Deploying SHIELD
By Larry Adams, C2ST Volunteer
As many as 50,000 students, faculty and staff on the campuses of the University of Illinois are tested twice a week for the COVID-19 virus, and a multimodal approach that the University developed has dropped positivity rates to less than a quarter of one percentage point.
The results are driving plans to roll out the program to communities across the state and beyond. Dubbed the SHIELD program, it was developed over the summer of 2020 by researchers and scientists at the University of Illinois’ Champaign-Urbana campus. SHIELD takes a three-pronged approach to mitigate the spread and the impact of the SARS-CoV-2 virus that causes COVID-19. The three legs of this approach are Target, Test and Tell, which is meant to determine who to test and how often, how to conduct the test, and how to disseminate the information.
During a recent C2ST Webinar, Deploying Shield to Illinois and Beyond. Jay Walsh, PhD, the University’s Vice President for Economic Development and Innovation, said the university was able to limit the spread of the virus through “social distancing and hygiene as well as a way of testing so that we can identify those who are infectious and remove them from the community by isolating them and quarantining their close contacts.”
Martin Burke, PhD, a professor and Associate Dean for Research at the University’s Carle Illinois College of Medicine, said that the SHIELD program’s data modeling determined that testing 50,000 people twice a week gave the team a “really good chance of catching people before they passed the disease onto others and thereby [we] have a good chance of stopping the spread.”
To accomplish this, a new testing approach was required to identify pre-symptomatic and asymptomatic carriers. The nasopharyngeal swab test was the most prevalent method available at the time, but it was not going to be sufficient to meet the University’s goals. “The swab method can be very uncomfortable and people don’t like to do that repeatedly,” Burke said during his Webinar presentation.
A team led by researcher Paul Hergenrother developed a new saliva-based test. The test uses saliva samples to test for the virus, as opposed to the nasopharyngeal method that uses a swab to collect a sample from the upper part of the throat, behind the nose.
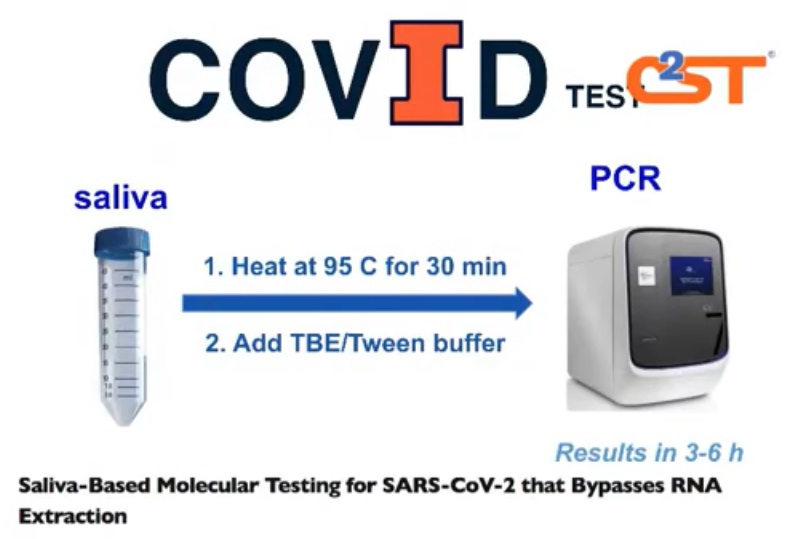
With the swab method, qualified medical professionals are required to capture the sample. The sample is then put into a viral transport medium, and later goes through goes through an RNA purification process. Once safe to work with, researcher put the sample through an RTQ-PCR reaction chamber, which amplifies the RNA to determine if the virus is present.
The breakthrough idea was to take the saliva sample and heat it at 95 deg. F for 30 minutes. The elevated heat level and duration time inactivates the virus making it safe for handling. Researchers also believe this thermal process breaks the sample “open” to expose the RNA allowing it to be directly transferred into the PCR, or polymerase chain reaction, reaction chamber.
“The process is very simple, very fast and very scalable,” Burke said.
The Shield testing program has reduced per test costs to about $20 to $30, much less than the $100 or more costs for a standard nasal swab test. In addition, results are learned much sooner. It typically takes 2 to 3 days to get the results from most currently available tests. The UofI saliva test notification time is 6 to 12 hours.
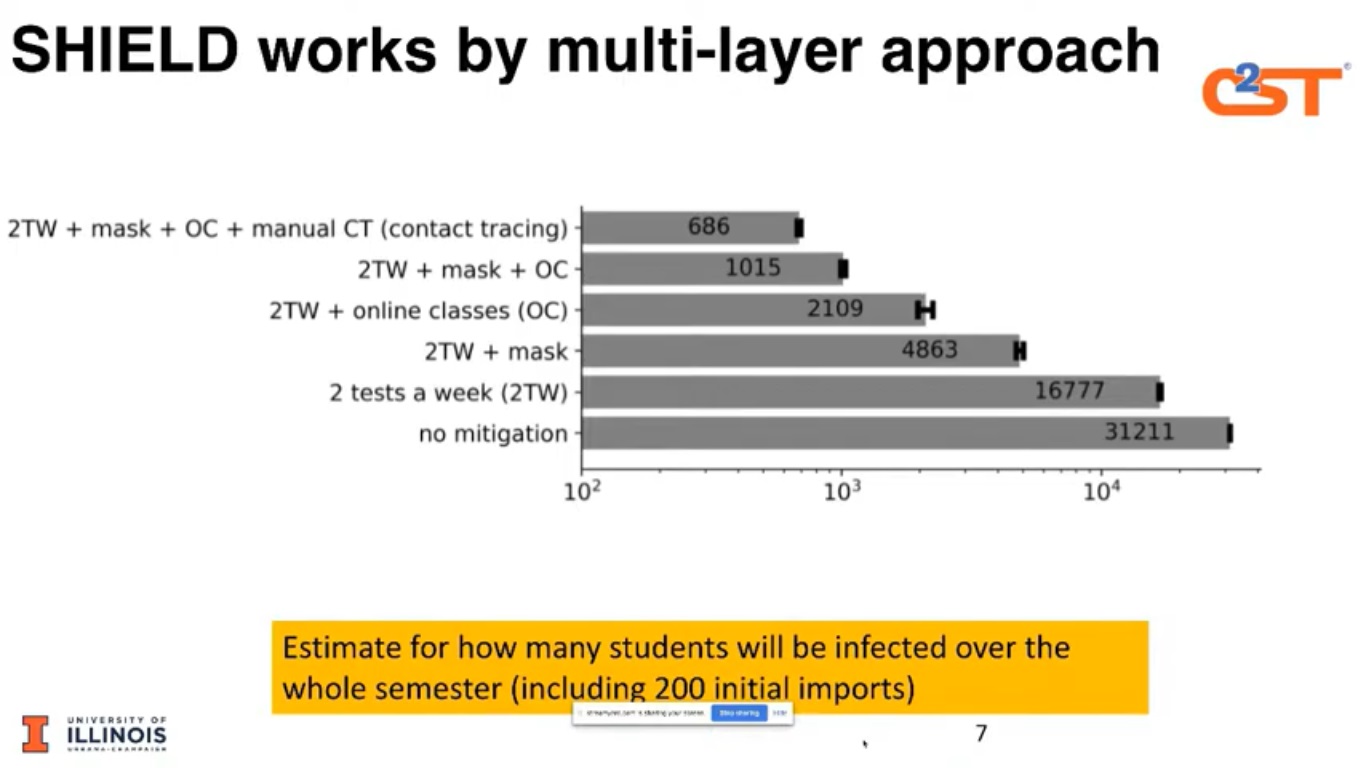
As of October, the school had conducted more than 500,000 tests on students’ faculty and staff. The testing process, as well as the targeting and telling approach, has helped reduce positivity rates to 0.24%. Equally important, if, and when, there is a surge, the outbreak is easier to identify, target and contain.
“What we clearly learned is that being nimble and looking at results in real time and responding to those
in real time is really important,” said Walsh.